Chirality: The Science of Handedness and How it Shapes Our World
Place your hands in front of you with your palms facing up, and you will see that your left and right hands are mirror images of each other. Now place your left palm on your right (with both palms still facing up), and you will observe that you cannot hide one hand behind the other completely. That is, they cannot be superimposed on each other. This is the defining characteristic of chirality: two objects are non-superimposable mirror images of each other.
Coming from the Greek word χείρ (kheir), which means “hand,” the phenomenon of chirality can be found all around us.
Chirality in Nature
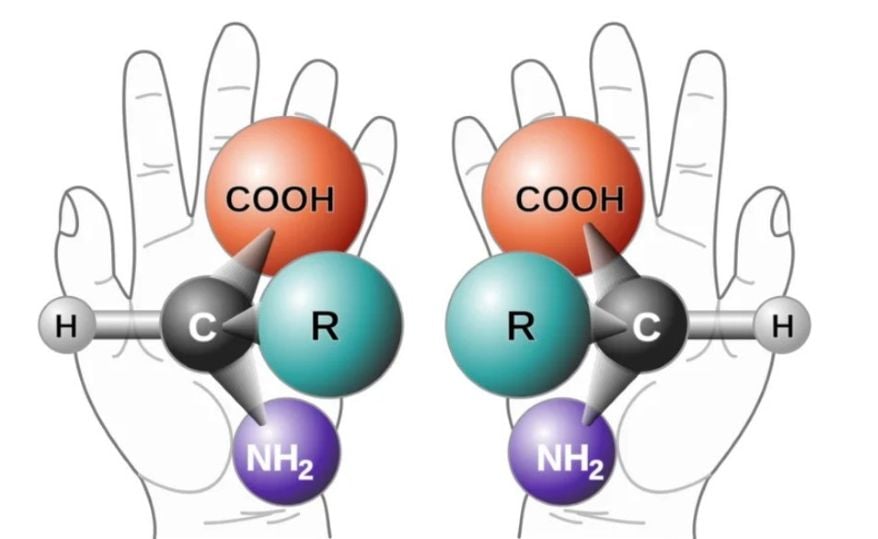
Chirality manifests in nature across all scales, from massive spiral galaxies to the shells of gastropods and snails, all the way down to molecules that make up life, like sugars, amino acids, and even DNA. Each of these exists in pairs that are mirror images of each other and are called enantiomers, and are usually designated as right-handed and left-handed depending on their appearance.
However, things become more complicated when we look at molecules, as their handedness is not immediately obvious, since the two forms have the same chemical properties. We can tell them apart by observing how they react with other chiral compounds. In biology, for instance, enzymes and receptors are chiral, and they often bind or ‘fit’ preferentially with only one chiral form of a substance, and not the other.


Homochirality and The Origin of Life
While both mirror images of a substance may be found in nature, in many instances, one form is more abundant. For example, DNA is helical and shows a single preferred handedness. Amino acids build proteins almost entirely, showing one kind of handedness, and sugars in our bodies show the same.
This uniform choice made by nature is called homochirality and is crucial for life to exist as it does today. How it all began is still an open question, with ideas ranging from tiny asymmetries in physics to circularly polarized light in space, to crystal surfaces that favor one hand, to self-amplifying reactions where a small imbalance grows over time. Once life picked a hand, biology kept it, because matching hands fit together.
Molecular Chirality in Action
A consistent method of identification of enantiomers is necessary, as choosing the wrong one can have undesirable consequences. This is because molecular chirality can affect our body in different ways, from smell and taste to causing more severe health effects. A classic example is limonene, a molecule found in citrus peels: one mirror form smells like oranges, and its twin smells like lemons.
Another, more cautionary case is that of thalidomide, where one enantiomer was widely prescribed as a cure for morning sickness. But its other form turned out to be highly toxic, leading chemists to rigorously test and control handedness, especially in pharmaceutical drugs.
So, it is essential to be able to distinguish between the two forms of chiral molecules. Fortunately, it is possible to do this just by observing how each enantiomer interacts with polarized light. In fact, you can try it yourself at home or at school.
A Classroom Activity
Try this quick activity with simple materials. Take two pairs of polarized sunglasses, stack the lenses, and slowly rotate one while looking at a bright screen or the sky. Hence, you will see the brightness change as the polarizers cross. Now, place a clear glass of sugar solution between the lenses.
Rotate again and look for a small shift in the angle where the light is darkest. The dissolved sugar causes that angular shift, called optical rotation. Congratulations, you have just measured molecular chirality with everyday tools! In fact, this was how the phenomenon of chirality was originally discovered.
Optical Rotation and Pasteur’s Discovery
In the 1840s, French scientist Jean-Baptiste Biot had already shown that some dissolved substances can twist the plane of polarized light. Hence, this behavior is called optical rotation. Building on that clue, a young Louis Pasteur noticed that tiny crystals of a tartrate salt came in two mirror-image shapes with left- and right-leaning faces.
He patiently separated the two kinds by hand, dissolved them, and found that each solution rotated polarized light, but in opposite directions. From this, he argued that the crystals were revealing an underlying molecular “handedness”. Further, he published work linking crystal form, chemical composition, and the sense of optical rotation. To persuade doubters, Pasteur even repeated the separation later at the Collège de France under Biot’s watchful eye.
Advanced Imaging Applications
The same optical rotation you measured with a sugar solution is now being used to make smarter cameras. Engineers use optically active crystals, such as quartz, that rotate polarized light by different amounts at different colors. Therefore, this lets a compact camera encode color information into polarization and then read it back with software.
The result is Spectro-polarimetric imaging, which can help biologists see more detail in microscopes, aid machine vision, and enable satellites or drones to monitor farms and forests. These systems already pick out stress patterns in plastics, distinguish human-made objects from vegetation, and even operate beyond visible light into the infrared for remote sensing and space exploration.
Chirality in Modern Technology
Chirality is hiding in plain sight. Just take a look at the device on which you are reading this article— maybe your phone, a tablet, or even a laptop. Moreover, many displays rely on polarization and chiral liquid crystals to control pixel brightness.
Some 3D movie systems use circularly polarized light and matching glasses so that each eye receives a different image. Engineers are also building chiral metamaterials that respond strongly to one circular polarization of light. Thus, boosting sensor signals, enhancing security features, and opening new ways to control light in compact devices.

Electron Spin and Chiral Molecules
Light is not the only thing that notices handedness. Electrons carry a property called spin that behaves like a tiny compass needle. Some chiral molecules prefer electrons with one spin over the other, a phenomenon known as chiral-induced spin selectivity. If we learn to control this effect reliably, chiral molecules could serve as room-temperature spin filters for next-generation sensors and low-power electronics.
Conclusion
Chirality ties together ideas from the lab bench, the clinic, the toolbox, and the sky. A mirror flip at the molecular scale can switch a scent from mint to caraway, change how a drug binds to a protein, or reveal which hand is present in a solution. Polarized light, from simple sugar solutions to advanced cameras, lets us see that hidden twist.
Life’s preference for one chiral form of amino acids and sugars reminds us that small imbalances can grow into rules that shape everything else. Once you learn to spot chirality, you will notice its twists in science, technology, and the world around you.
References
- Blackmond, D. G. (2010). The origin of biological homochirality. Cold Spring Harbor Perspectives in Biology, 2(5), a002147. https://doi.org/10.1101/cshperspect.a002147
- Cao, Z., Sun, S., Wei, J., & Liu, Y. (2025). Dispersive optical activity for spectro-polarimetric imaging. Light: Science & Applications, 14, 90. https://doi.org/10.1038/s41377-025-01766-5
- Ceramella, J., Iacopetta, D., Franchini, A., De Luca, M., Saturnino, C., Andreu, I., Sinicropi, M. S., & Catalano, A. (2022). A Look at the Importance of Chirality in Drug Activity: Some Significative Examples. Applied Sciences, 12(21), 10909. https://doi.org/10.3390/app122110909
Additionally, to stay updated with the latest developments in STEM research, visit ENTECH Online. Basically, this is our digital magazine for science, technology, engineering, and mathematics. Furthermore, at ENTECH Online, you’ll find a wealth of information.


