Quantum Interactions: Tale of Colors in Fruits and Vegetables
The vivid colors of fruits and vegetables arise from quantum interactions or effects in pigment molecules. Conjugated π-electron systems in pigments like beta-carotene and chlorophyll absorb specific wavelengths of visible light via electronic transitions. Moreover, using the particle-in-a-box model, we can estimate these transitions and link them to observed colors. Hence, this results from unabsorbed, complementary wavelengths.
The year 2025 has been designated the International Year of Quantum Science and Technology (IYQ 2025). Celebrating one of the most profound and transformative fields in human knowledge. However, quantum physics, once seen as an abstract and highly mathematical domain, now forms the backbone of many modern technologies. Indeed, from lasers and semiconductors to MRI scanners and quantum computers, quantum phenomena govern some of the most fundamental natural processes. Mostly, we encounter that in everyday life.
When photons encounter matter, their energy determines whether they are absorbed or reflected. Therefore, processes are governed by quantum mechanics. Also, in the eye, photoreceptors convert these interactions into neural signals, allowing the brain to perceive color. From carotenoids in carrots to anthocyanins in berries, nature’s palette reflects the deep connection between quantum physics and human vision. Hence, a fitting tribute to the spirit of IYQ 2025. Further, this article explores how the vibrant colors of fruits and vegetables arise from quantum interactions between light and molecular pigments.
Process of Color Perception
The vivid colors of fruits and vegetables result from how their pigments absorb and reflect light. Eventually, when light strikes an object, some wavelengths are absorbed by the material, while others are reflected. However, the specific pigments in fruits and vegetables, such as chlorophyll in spinach or beta-carotene in carrots, selectively absorb certain parts of the visible spectrum. Moreover, based on their molecular structure.
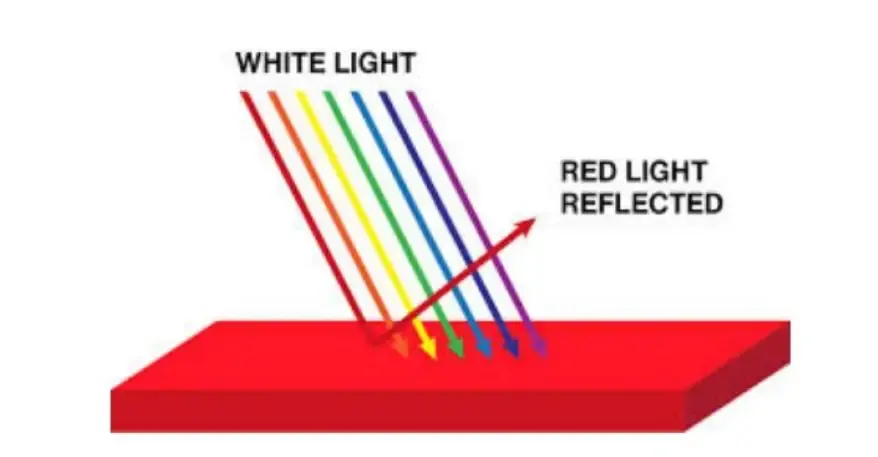
Therefore, the colors we perceive are those wavelengths that are not absorbed but reflected to our eyes. For example, a tomato appears red because its pigments absorb most other colors and reflect red light. Thus, the striking hues of natural produce are shaped by the selective absorption. Also, the reflection of light by plant pigments, interpreted by our visual system.
Pigments Present in Fruits and Vegetables
1. Chlorophylls–
- Color: Green
- Found in: Leafy Greens (Spinach, Kale, Parsley)
- Types and Formulas: – Chlorophyll a: C55H72MgN4O5 and Chlorophyll b: C55H70MgN4O6
2. Carotenoids–
- Colors: Orange, Red, Yellow
- Found in: Carrots, Tomatoes, Corn
• Examples:
- Beta-carotene: C40H56
- Lycopene: C40H56
- Lutein: C40H56O2
- Zeaxanthin: C40H56O2
3. Anthocyanins–
- Colors: Red, Purple, Blue
- Found in: Berries, red cabbage, grapes
- Example : Cyanidin-3-glucoside: C21H21O11
4. Betalains–
- Colors: Red-violet, Yellow-orange
- Found in: Beets, Swiss chard, amaranth
- Examples:
– Betanin: C24H26N2O13
– Indicaxanthin: C19H21N3O9
5. Flavonoids–
- Colors: Light yellow or colorless
- Found in: Apples, onions, citrus peels
- Example: Quercetin: C15H10O7
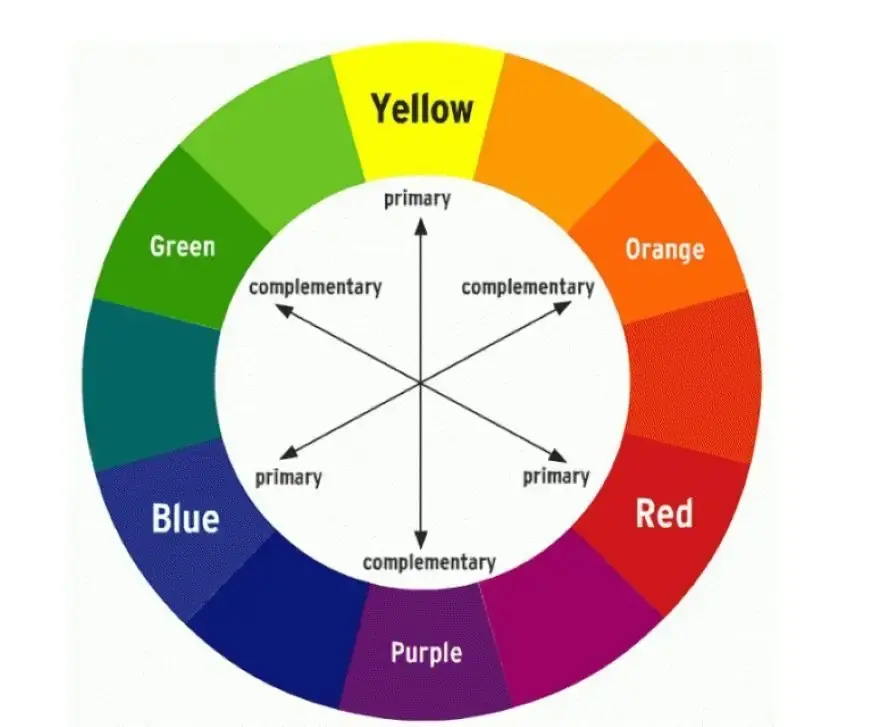
Complementary Colors
When a substance absorbs light of a certain wavelength, the color perceived by the human eye is its complementary color. Indeed, it is the color opposite on the color wheel.
- Blue absorption (∼450 nm) appears orange
- Green absorption (∼510 nm) appears magenta
- Red absorption (∼650 nm) appears cyan
Quantum Physics Behind the Colour of Carrot
Carrots appear orange due to ![]() , which absorbs blue light. Further, we can model its electronic structure using the particle-in-a-box approximation:
, which absorbs blue light. Further, we can model its electronic structure using the particle-in-a-box approximation:
![]()
With 22 delocalized ![]() -electrons (from 11 double bonds), HOMO
-electrons (from 11 double bonds), HOMO ![]() , LUMO
, LUMO ![]() . Thus, the transition energy is:
. Thus, the transition energy is:
![]()
With ![]() :
:
![]() =
= ![]()
![]()
= ![]()
Hence, the wavelength is:![]()
= ![]()
= ![]()
Therefore, this matches the absorption of blue light, hence the carrot appears \textbf{orange}.
Why Carrot Juice Changes Color Over Time
The orange color of carrot juice fades over time due to the degradation of beta-carotene and other pigments. Eventually, oxidation, driven by exposure to oxygen, disrupts the conjugated double bonds in beta-carotene. Hence, forming colorless or brown degradation products. Further, ultraviolet light accelerates photodegradation, converting the more vibrant trans-isomers into less colorful cis-isomers. Moreover, heat further breaks down beta-carotene into less pigmented or colorless compounds. Additionally, enzymatic activity, particularly from polyphenol oxidase (PPO), oxidizes phenolic compounds naturally present in the juice. Thus, leading to browning and a loss of the characteristic orange hue.

Conclusion
The vivid colors of nature — from fruits and vegetables to flowers — arise from quantum interactions within molecular pigments. Therefore, through selective light absorption by conjugated systems, quantum mechanics paints the world in complementary hues. Moreover, it reveals the invisible physics behind everyday beauty.
References
- Khoo, H., Prasad, K. N., Kong, K., Jiang, Y., & Ismail, A. (2011). Carotenoids and their isomers: color pigments in fruits and vegetables. Molecules, 16(2), 1710–1738. https://doi.org/10.3390/molecules16021710
- Zielinska, M., & Markowski, M. (2011). Color characteristics of carrots: Effect of drying and rehydration. International Journal of Food Properties, 15(2), 450–466. https://doi.org/10.1080/10942912.2010.489209
- Blumfield, M., Mayr, H., De Vlieger, N., Abbott, K., Starck, C., Fayet-Moore, F., & Marshall, S. (2022). Should we ‘Eat a Rainbow’? An umbrella review of the health effects of colorful bioactive pigments in fruits and vegetables. Molecules, 27(13), 4061. https://doi.org/10.3390/molecules27134061
Additionally, to stay updated with the latest developments in STEM research, visit ENTECH Online. Basically, this is our digital magazine for science, technology, engineering, and mathematics. Further, at ENTECH Online, you’ll find a wealth of information.






